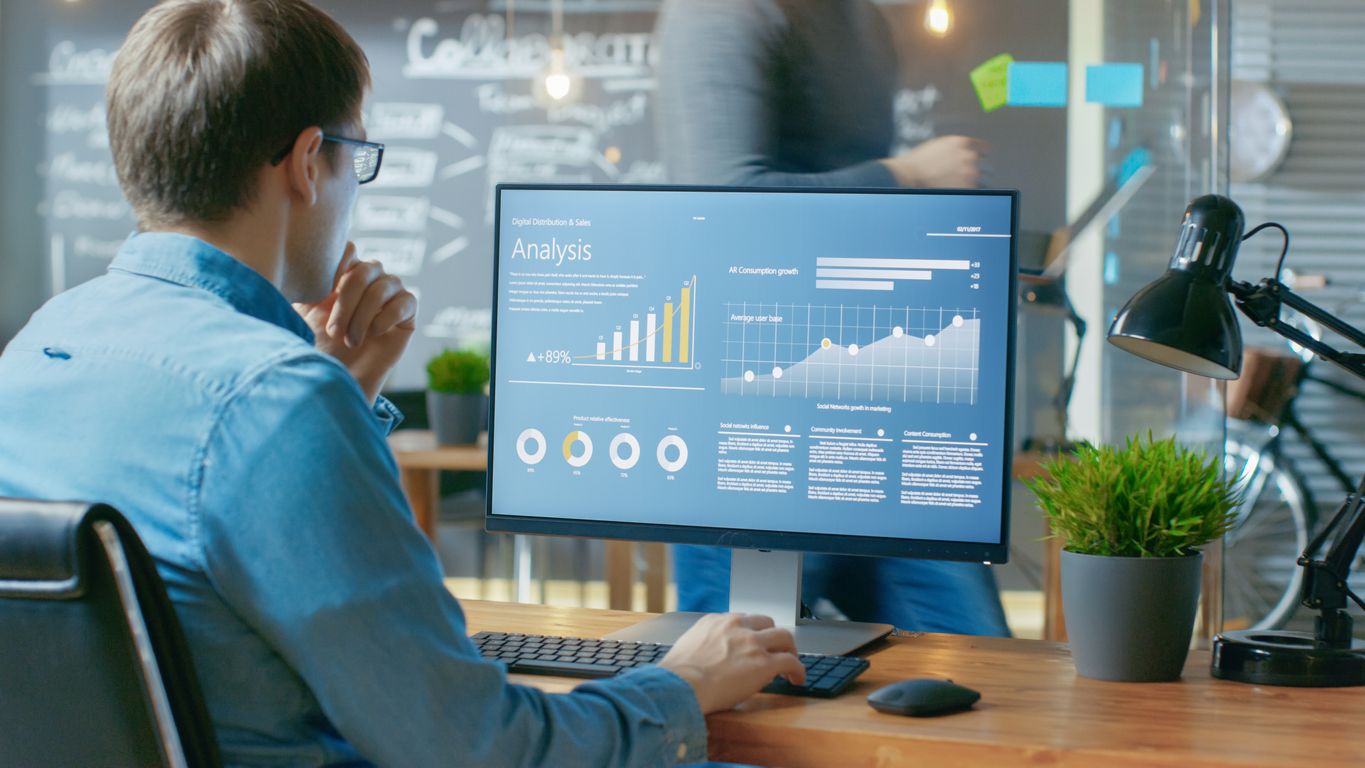
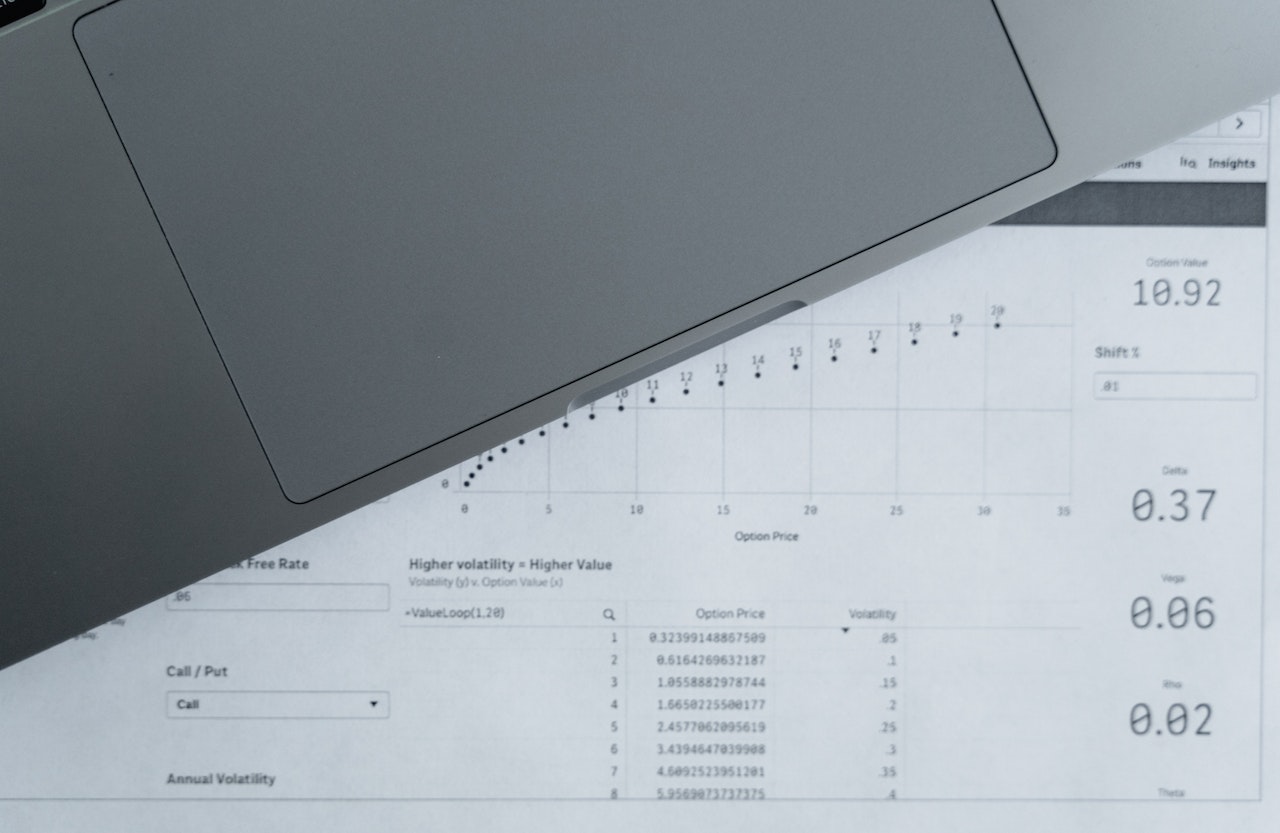
Mother your children are like birds
Verse 1 For as long as I can remember, The windows always glowed for me, In the room filled with quiet spring, And embroidered towels on the wall. In that sacred, peaceful chamber, A child’s heart would read and know Shevchenko’s kind and watchful eyes, And golden...
1win официальный сайт букмекерской конторы и онлайн-казино 1Вин
1Win – это популярная онлайн платформа в России, которая сочетает в себе возможности ставок на спорт и азартных игр. Здесь пользователи могут делать ставки на широкий спектр спортивных событий, включая как традиционные виды спорта, так и киберспорт. В разделе казино...
A mesterséges intelligencia hatása a kaszinó műveletekre
A mesterséges intelligencia (AI) megváltoztatja a kaszinó szektorot azáltal, hogy javítja a műveleteket és javítja a vendég interakciókat. 2023 -ban egy tanácsadó cég dokumentuma kiemelte, hogy az AI eszközök akár 30%-kal is növelhetik a munkahelyi hatékonyságot,...
The Evolution of Casino Loyalty Programs
Casino loyalty programs have experienced significant changes over the years, transforming from simple punch passes to complex digital systems. These programs are designed to reward recurrent visitors with various advantages, including complimentary play, food, and...
Il Futuro dei Casinò: Innovazioni e Tendenze
Negli finali periodi, l’industria dei giocodromi ha sostenuto una trasformazione importante grazie all’adozione di sistemi innovative. Stando a un documento del anno duemilaventitre di Statista, il mercato globale dei casinò online è previsto crescere a un percentuale...
The Rise of Online Casinos: Trends and Insights
The online casino field has witnessed significant growth over the past few periods, particularly hastened by the global pandemic. In 2022, the online betting market was estimated at roughly (63 billion and is projected to reach )114 billion by 2028, in accordance to a...
O impacto da inteligência artificial nas operações do cassino
Inteligência artificial (IA) está transformando a indústria de jogos de azar, refinando operações, aprimorando as experiências dos clientes e atualizando estratégias de segurança. Em 2023, um relatório da Deloitte enfatizou que as ferramentas de IA poderiam aumentar a...
The Evolution of Casino Loyalty Programs
Casino loyalty initiatives have changed considerably over the years, developing from simple validation cards to sophisticated digital platforms that track participant behavior and likes. These schemes are created to compensate regular guests with multiple benefits,...
Nowe Technologie w Kasynach Online
W ostatnich latach kasyna online zyskały na fame dzięki implementacji zaawansowanych technologii, które zmieniają sposób, w który gracze uczestniczą w grach hazardowych. W 2023 roku kwota rynku gier online osiągnęła 70 miliardów dolarów dolarów, a prognozy sugerują na...
Casino Bonus Bez Depozytu Bonus Bez Depozytu Za Rejestrację
Warunki promocji bez depozytu są takie same we wszystkich kasyno online. Zanim zaczniesz grać w kasyno online, musisz zrozumieć zasady i warunki, zwłaszcza te dotyczące bonusów bez depozytu. Zarówno nowi, jak i obecni gracze mogą otrzymać bonus bez depozytu na stronie...
The Role of EDC in Oncology Clinical Trials
In the evolving landscape of oncology clinical trials, data management plays a pivotal role in ensuring the accuracy, integrity, and timeliness of research. Electronic Data Capture (EDC) systems have transformed how data is collected, stored, and analyzed, offering a...
How EDC Systems Streamline Clinical Data Management
In today’s rapidly evolving healthcare and clinical research environment, the need for efficient, accurate, and compliant data management solutions is paramount. Clinical trials generate vast amounts of data, and the process of managing, storing, and analyzing this...
The Role of Third-Party Audits in EDC Validation
In the fast-paced world of clinical trials, data integrity is crucial for ensuring the validity of trial results.Electronic Data Capture (EDC) systems are pivotal in this process, as they enable the collection, management, and analysis of clinical trial data.However,...
The Benefits of Using ePRO-EDC Solutions in Patient-Reported Outcomes
In the ever-evolving landscape of clinical trials, Patient-Reported Outcomes (PROs) play a crucial role in assessing the efficacy of treatments and therapies from the patients' perspectives. However, the traditional methods of capturing these outcomes have often been...
The Role of EDC in Managing Patient Registries
In the ever-evolving landscape of healthcare and clinical research, managing patient data efficiently and accurately is a top priority.Patient registries, which collect and store data about individuals with specific health conditions, play a critical role in...
Best Practices for Using EDC in RWE Studies
In the realm of clinical research, Real-World Evidence (RWE) studies have become an essential component in shaping healthcare decisions and improving patient outcomes. RWE studies utilize real-world data (RWD) collected from sources like electronic health records,...
The Role of EDC in Multi-Institutional Academic Studies
Multi-institutional academic studies are critical for advancing scientific research, as they bring together diverse expertise, resources, and perspectives across various institutions.However, managing and coordinating data from multiple institutions can be a daunting...
Understanding 21 CFR Part 11 Compliance for EDC Systems
In clinical trials, the accuracy and integrity of data are paramount to ensuring the safety and efficacy of new medical treatments.Regulatory bodies, particularly the U.S. Food and Drug Administration (FDA), impose stringent standards to safeguard this data, and one...
Cost-Effective EDC Solutions for Small-Scale Clinical Studies
In the world of clinical trials, data management plays a critical role in ensuring the accuracy, integrity, and efficiency of the study process.With the rising costs of clinical trials, especially in smaller-scale studies, managing expenses becomes a priority without...
The Importance of EDC in Clinical Trials
In the fast-evolving world of clinical trials, data accuracy, speed, and security are critical factors for successful research and development. Electronic Data Capture (EDC) systems have emerged as a cornerstone of modern clinical trials, fundamentally changing the...
Best Practices for Using EDC in Oncology Studies
In the ever-evolving world of oncology research, electronic data capture (EDC) systems have become invaluable tools. EDC solutions help streamline data collection, enhance accuracy, and improve the efficiency of clinical trials. However, the unique challenges...
The Role of EDC in Academic Clinical Research
In the rapidly evolving world of clinical research, the need for accurate, efficient, and transparent data collection has never been more critical. Electronic Data Capture (EDC) systems have emerged as a game-changer in the field of academic clinical research,...
Affordable EDC Solution for Patient Registries
In the ever-evolving landscape of medical research, patient registries have emerged as a cornerstone for collecting real-world data, enabling researchers to track patient outcomes, monitor disease progression, and design more effective clinical trials.However, the...
Low-Cost EDC Software for Academic Clinical Research
If you need an EDC system solution, please contact us at info@www.klindat.com Clinical research is the backbone of medical advancement, driving innovations in treatments, diagnostics, and patient care. However, the success of any clinical study hinges on the ability...
Guide to EDC Systems for Clinical Trials 2025
If you need an EDC system solution, please contact us at info@www.klindat.com Electronic Data Capture (EDC) systems have become an indispensable tool in modern clinical trials, offering a more efficient, accurate, and secure way to collect and manage clinical trial...
The Evolution of Electronic Patient-Reported Outcomes (ePRO) and Data Collection
If you need an ePRO solution, please contact us at info@www.klindat.com In the realm of clinical trials and patient registries, the use of Electronic Patient-Reported Outcomes (ePRO) has gained significant traction in recent years.The advancements in technology have...
Effective Clinical Database Locks: Ensuring Data Integrity and Reliability
If you need clinical data management services, please contact us at info@www.klindat.com The integrity and reliability of the data collected in clinical trials are of utmost importance.Database lock (DBL), also known as data lock, is a critical step in the data...
Effective eCRF Design: A Comprehensive Guide
If you need support to design an eCRF, please contact us at info@www.klindat.com In the world of clinical research, data collection is the linchpin of successful studies.And at the heart of data collection is the electronic case report form (eCRF).Designing an...
The Essential Role of Audit Trails in EDC Systems
If you need an EDC system with a solid audit trail module, please contact us at info@www.klindat.com In today's digital world, the use of electronic data capture (EDC) systems has become increasingly prevalent in clinical research.These systems play a vital role in...
Why Using EDC Systems in Clinical Trials
If you need an EDC system for a clinical trial, please contact us at info@www.klindat.com Electronic Data Capture (EDC) systems have revolutionized the way clinical trials are conducted in the modern healthcare industry.These powerful software tools enable researchers...
The Evolution of EDC in Post-Marketing Studies
Do you need an EDC system for a post-marketing study? Contact us at info@www.klindat.com In the world of pharmaceuticals, ensuring the safety, effectiveness, and efficacy of new drugs and therapies is of utmost importance.Clinical trials are conducted prior to product...
EDC Integration with Other Systems: Enhancing Clinical Research Efficiency and Data Management
If you need an EDC system that can be integrated with other applications, please contact us at info@www.klindat.com In the rapidly evolving field of clinical research, the incorporation of Electronic Data Capture (EDC) with other cutting-edge technologies has emerged...
Patient Disease Registry Software: Enhancing Healthcare Outcomes
If you need a disease registry software application, please contact us at info@www.klindat.com In the rapidly evolving landscape of healthcare, patient disease registries have emerged as powerful tools for improving patient outcomes and advancing medical...
Electronic Patient-Reported Outcome (ePRO) Apps in Breast Cancer Care
Please contact us at info@www.klindat.com if you need an ePRO application for a breast cancer studyBreast cancer is a prevalent disease affecting one in eight women during their lifetime [1].As the field of cancer therapy continues to advance, there is a growing...
Viability of ePRO for Older Patients with Cancer
This article was written based on the data reported in “Usability of Electronic Patient-Reported Outcome Measures for Older Patients With Cancer: Secondary Analysis of Data from an Observational Single Center Study”If you need an ePRO application for cancer studies,...
Feasibility of ePRO Among Cancer Patients
This article has been written based on the information available at “Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home”If you need a cost-efficient ePRO application for cancer studies, please contact us at...
ePRO Surveys: Improving Patient Engagement and Data Collection
If you need an affordable ePRO software application, please contact us at info@www.klindat.comIn the world of clinical trials and healthcare treatments, patient-reported outcomes (PROs) hold great importance.These outcomes are based on the direct self-reporting of...
How to Choose the Right EDC Vendor for Your Clinical Study
Please contact us at info@www.klindat.com if you need an EDC vendor for your clinical studyChoosing the right Electronic Data Capture (EDC) vendor for your clinical study is a key decision that can impact the efficiency, accuracy, and success of your research.With...
Patient Registries in Oncology: A Comprehensive Guide
If you need a cancer patient registry software to collect and clean clinical data, please contact us at info@www.klindat.com Cancer is a complex disease that affects millions of people worldwide.Understanding the incidence, prevalence, and outcomes of different types...
The Importance of Patient Registries in Clinical Research
Please contact us at info@www.klindat.com if you need an EDC software application to implement a patient registryPatient registries play a key role in advancing medical research and improving patient care.These organized systems collect and analyze data about patients...
How Cardiovascular Registries Improve Patient Outcomes
If you need a software application to implement a patient registry, please contact us at info@www.klindat.com Cardiovascular disease is a significant health concern that encompasses conditions such as heart disease, stroke, and other disorders of the circulatory...
EDC Systems for Clinical Trials: Features and Benefits
If you need an EDC system for a clinical trial, please contact us at info@www.klindat.com Clinical trials play a major role in advancing medical research and improving patient care.To effectively manage and streamline the data collection process in clinical trials,...
Clinical Data Validation: Ensuring Accuracy and Quality in Clinical Trials
If you need an Electronic Data Capture (EDC) system or data management services for your clinical trial, please contact us at info@www.klindat.com Clinical trials are an essential part of medical research, providing valuable insights into the effectiveness and safety...
Choosing the Right Real-World Evidence Electronic Data Capture (EDC) Software
Please contact us at info@www.klindat.com if you need an EDC application for a real-world evidence studyIn recent years, the field of medical care and biomedical research has witnessed a data revolution.This transformation has been made possible through the use of...
The Value of Real-World Data in Healthcare Research
If you need a software application to collect real-world data, please contact us at info@www.klindat.com Real-world data (RWD) has become increasingly important in healthcare research, providing valuable insights into patient outcomes and therapy effectiveness.These...
How an EDC System Saves Costs in Clinical Trials
Please contact us at info@www.klindat.com if you need an EDC system for your clinical trialElectronic Data Capture (EDC) systems have revolutionized the way clinical research and pharmaceutical drug development are conducted.These systems streamline the data capture...
An Affordable EDC System for Clinical Trials
If you need an affordable EDC system for your clinical trial, please contact us at info@www.klindat.com In the world of clinical trials, the adoption of Electronic Data Capture (EDC) systems has revolutionized the way data is collected, managed, and exported.These...
Data Cleaning in Clinical Trials: Ensuring Accuracy and Regulatory Compliance
If you need data management services for clinical trials, you can contact us at info@www.klindat.com Clinical trials play a decisive role in the development of new drugs and medical devices that can improve patient outcomes and quality of life.However, the success of...
Why is CDASH Needed in Clinical Trials?
If you need support to implement the CDASH standard in your CRF, you can contact us at info@www.klindat.com Clinical trials are essential in the development and evaluation of new drugs and therapies.The success of these trials relies heavily on the collection,...
9 Tips to Design Better Case Report Forms (CRFs) for Clinical Trials
If you need support to design a CRF, please contact us at info@www.klindat.com Case report forms (CRFs) are an essential tool in clinical research for collecting patient data and ensuring the integrity of clinical trial outcomes.When designing CRFs, sponsors and...